Cover Picture
Nuclear magnetic resonance-guided Monte Carlo/molecular dynamics structure inference for amorphous solids
Kangren Kong, Zaiqiang Ma, Yu Yin, Zhaoming Liu, Ruikang Tang* Submit a Manuscript
Nuclear magnetic resonance-guided Monte Carlo/molecular dynamics structure inference for amorphous solids
Kangren Kong, Zaiqiang Ma, Yu Yin, Zhaoming Liu, Ruikang Tang* Submit a Manuscript
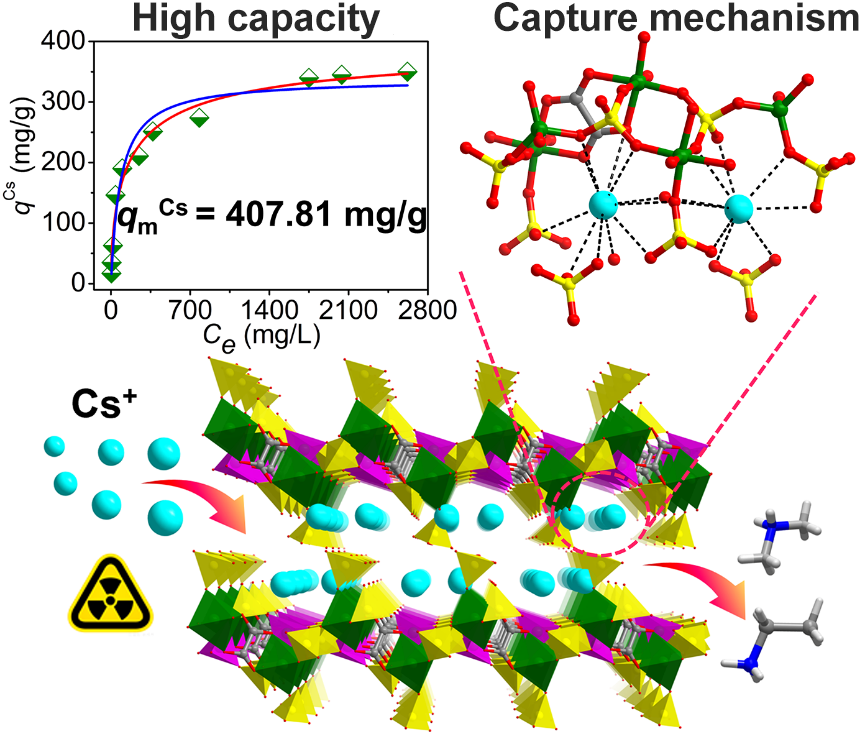
Efficient uptake of Cs+ by a layered gallium oxalatophosphate with the clear insight into remediation mechanism
Wen Ma*, Tian-Yu Pan, Tian-Tian Lv, Yan-Ling Guo, Hai-Yan Sun, Rui-Ping Yang, Jun-Hao Tang, Ting-Hui Zhuang, Kai-Qiang Jing, Mei-Ling Feng*, Xiao-Ying Huang
Chin. J. Struct. Chem., 2026, 45(3), 100829. DOI: 10.1016/j.cjsc.2025.100829
March 1, 2026
Cesium removal; layered materials; oxalatophosphate; ion exchange; structural transformation
ABSTRACT
It is of vital importance to effectively capture?137Cs for human health and ecological protection due to its strong radioactivity and biotoxicity. Herein, the efficient uptake of Cs+?has been achieved by a new layered gallium oxalatophosphonate {[(CH3)2NH2][CH3CH2NH3]}2[Ga4(PO4)4(H2PO4)2(C2O4)] (FJSM-NGAPC), whose structure features the anionic gallium oxalatophosphate layer of [Ga4(PO4)4(H2PO4)2(C2O4)]n4n-?with [(CH3)2NH2]+?and [CH3CH2NH3]+?cations in the interlayer spaces. The maximum Cs+?adsorption capacity of FJSM-NGAPC can reach 407.81 mg/g, which surpasses that of common Cs+?scavengers. In the presence of a large excess of interfering Na+?ions, it shows high selectivity for Cs+?ions and the maximum?KdCs?can reach 1.36 × 104?mL/g. In particular, FJSM-NGAPC can maintain removal performance for Cs+?in the pH range from 3.07 to 10.01 with?KdCs?values all above 103?mL/g. Impressively, the Cs+?adsorption mechanism is clearly revealed at the molecular level by the single crystal to single crystal (SC-SC) structural transformation. This process confirms ion exchange between Cs+?and interlayer [(CH3)2NH2]+?and [CH3CH2NH3]+?cations, accompanied by strong Cs···O interactions. This work provides an efficient metal oxalatophosphate as the scavenger for radiocesium and clearly elucidates the radiocesium capture mechanism, facilitating the design of new oxalatophosphates materials for radionuclide remediation.